Introduction:
In multiple myeloma (MM), plasma cells including malignant cells show expression of CD38, which is the target for daratumumab (Dara), an IgG1k monoclonal antibody, approved for MM treatment. This review highlights the efficacy and safety of Dara addition to the standard of care therapy in newly diagnosed MM (NDMM) patients (pts).
Methods:
We conducted a literature search using four databases (PubMed, Embase, Web of Science, and Clinicaltrials.gov). Our search strategy included MeSH terms and key words for multiple myeloma and Dara including trade names and generic names from date of inception to April 2020. A total of 587 articles were screened, and after excluding review articles, duplicates, and non-relevant articles, we included three phase III trials reporting daratumumab addition to the standard of care regimens for NDMM patients.
Results:
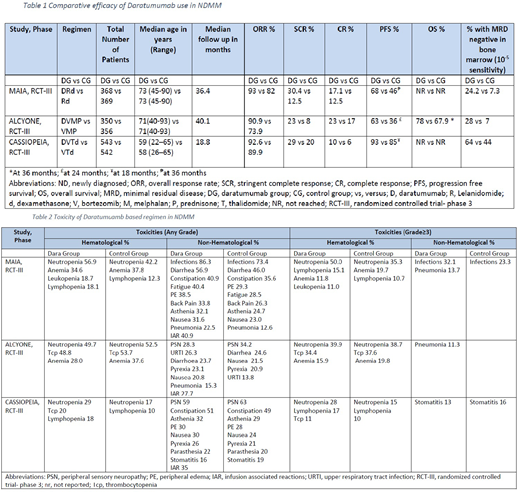
A total of 2528 patients were enrolled and evaluated in three phase III randomized controlled trials. A total of 1261 patients were in the daratumumab group and 1267 patients were in the control group. Total 1085 were transplant eligible and 1443 patients were transplant ineligible.
Transplant eligible:
Moreau et al. (2019) underlined the efficacy of Dara + bortezomib (V) + thalidomide (T) + dexamethasone (d) in NDMM pts (n=1085) in CASSIOPEIA phase III trial. Addition of Dara to VTd group showed marked improvent in progression free survival (PFS): 93% vs 85% at 18 months (hazard ratio (HR) 0.42 [0.34-0.51]; p<0.0001), and overall response rate (ORR) of 92.6% (CI: 95%, P <0.0001) in Dara + VTd group as compared to VTd group (89.9%). Minimal residual disease (MRD) negative status (10-⁵ sensitivity threshold, assessed by multipara metric flow cytometry) in bone marrow was 64% (P<0.0001) in Dara using VTd vs 44% in VTd group.
Transplant ineligible:
Bahlis N et al. (2019) studied Dara + lenolidamide (R) and dexamethasone (d) in NDMM pts (n=737) in MAIA phase III trial. Mateos et al. (2018) reported the role of Dara + V + melphalan (M) + prednisone (P) vs VMP in NDMM pts (n=706) in a phase III trial (Alcyone). Addition of Dara to Rd, and VMP group showed marked improvent in PFS: 68% vs 46% at 36 months [confidence interval [CI], 0.44-0.71; P<0.0001], and 63% vs 36 at 24 months (HR: 0.47, 95% CI 0.33-0.67, p<0.0001), and ORR: 93% vs 82% (P<0.0001), and 90.9% vs 73.9%, respectively. MRD negative status in bone marrow was observed higher with Dara compared to without Dara using Rd (24.2% vs 7.3%) (P<0.001), and Dara + VMP group (28% vs 7%) (p<0.0001) respectively.
Significant toxicities mainly included pancytopenias and opportunistic infections (table 2). Standard care regimen (VTd, VMP, Rd) with Dara addition achieved superior outcomes in terms of ORR and PFS. Addition of Dara improved MRD negative status.
Conclusion:
NDMM treatment with Dara plus standard care therapy (VTd, VMP, Rd) has shown promising outcomes and has set a benchmark for future studies.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal